Particle size determination in metal oxides
A Lens to the Nanoscale - Precision Measurement of Ultrafine Particles
Belkacem El Idrissi, Analytical Lab Manager at AEM Canada
Introduction
The characterisation of high purity alumina (HPA) particles
is principally achieved through wet laser diffraction particle
size analysis, a technique capable of providing excellent
insight into the particle size distribution (PSD), typical
known as D10, D50 and D90 fractions, of alumina in either
powder or slurry form.
The choice of measurement technique often depends on the end user’s requirements. It is very important for the recipient to determine their application and needs in terms of particle size distribution; either wet or dry. This will help us to identify the most suitable method that best represents the target application.
The choice of measurement technique often depends on the end user’s requirements. It is very important for the recipient to determine their application and needs in terms of particle size distribution; either wet or dry. This will help us to identify the most suitable method that best represents the target application.

The typical challenge in the determination of particle size is
linked to the fact that fine particles tend to agglomerate,
like in Fig. 1, and stick to each other via Van der Waals
bonding; which means they are relatively easy to break into
smaller particles via non-intrusive agitation methods such
as sonication. This means that sometimes the operator
measures the size of the agglomerates and not the primary
size of the particles. The measurement of the true particle
size dimensions by laser diffraction can present a number
of challenges, in particular interpreting the different results
obtained by different characterisation techniques or
methods (SEM imaging) or particle size distribution (PSD)
measurements in a coherent manner. This article will
address the main difficulties associated with measuring
alumina agglomerates by laser diffraction (wet route)
during the process of wet grinding, drying of the alumina
suspension and dry re-grinding of the clogged alumina to
the size desired by our customers. ‘
Challenges in Measuring the PSD of Sub-micron Alumina Agglomerates:
As said before. the distribution and size of finely ground
alumina particles is often affected by the presence of
agglomerates, linked to ripening, aggregation or
agglomeration phenomena. Agglomeration of alumina
nanoparticles can have a significant impact on PSD results.
Understanding these phenomena and controlling the
complete and uniform dispersion of solid particles will
enable us to determine whether additional dispersive
energy (agitation, heating, sonication, etc.) is required to
obtain representative results for the product. Otherwise
there is a risk of obtaining erroneous distribution results,
i.e. an overestimate of particle size and/or an
underestimate of the number of fine particles in the
sample.
In order to choose the right measurement method, it is
very important to take SEM or optical microscope images to
qualitatively characterise the shape of the particles and to
assess the rate and type of agglomeration of the alumina
particles (Figure 2). It should be noted that SEM is a
qualitative technique that in no way provides reliable
statistics or results on particle size. It is therefore essential
to use a suitable technique to measure the size distribution
of fine particles or nanoparticles (submicron alumina). The
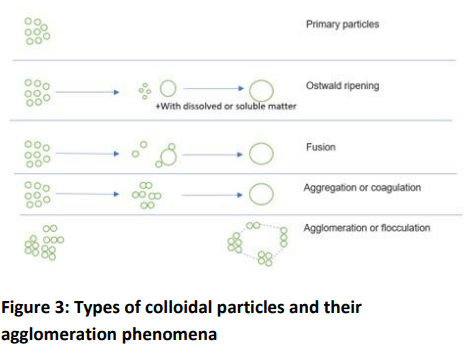
process of agglomeration is very complex and it is
summarized in Figure 3.

Ripening – A phenomenon which depends on the solubility
of alumina in water. Smaller particles have a tendency to
present more surface deformation, resulting in higher
surface energy and increased solubility. The preferential
solubility of small particles will be greater than that of
larger particles. Over time, the population of small
particles will dissolve in solution. The dissolved part of the
small particles will then be deposited on the larger
particles, and the alumina particles will become larger. This
process is known as Ostwald ripening.
Aggregation – Primary particles can come into contact,
sticking together to form strong chemical bonds over a
small area or point of contact. The mechanism is called
aggregation or coagulation. Aggregates of very small or few
primary particles can still be found in the colloidal size
range. Because of the bonds between the primary
particles, they are usually quite difficult to separate using
simple techniques such as stirring, heating or sonication.
Agglomeration – Agglomeration is a phenomenon whereby
fine alumina particles adhere to each other to form larger
particles by means of intermolecular bonding and are often
measured as a single larger particle. Unlike individual
smaller particles that form agglomerate, this type of
agglomeration is generally easy to break up by simple
means such as agitation, heating or sonication.
Agglomeration of fine particles can also affect the shape
and size distribution of particles.
A primary particle is the smallest identifiable subdivision in
a particulate system. Primary particles can stick together to
form “soft/fragile” (easily dispersed) or “hard” (fused)
agglomerates.
The stability of a colloidal system or suspension depends on a number of factors including pH, ionic strength, temperature and the ‘history’ of the dispersion, because in many suspensions the colloid ‘remembers’ its history from the primary particle.
Finely ground metal oxides, and more specifically alumina, are materials that tend to form agglomerates, which means that individual fine particles can come together to form larger structures.
The stability of a colloidal system or suspension depends on a number of factors including pH, ionic strength, temperature and the ‘history’ of the dispersion, because in many suspensions the colloid ‘remembers’ its history from the primary particle.
Finely ground metal oxides, and more specifically alumina, are materials that tend to form agglomerates, which means that individual fine particles can come together to form larger structures.
The agglomeration of metal oxides particles can be caused
by several factors, such as the physio-chemical
characteristics of the surface of fine alumina particles (pH,
conductivity, etc.), electrostatic forces, Van der Waals
forces (attraction and repulsion), micronisation (grinding)
and certain storage conditions can also make these
particles (generally <10 µm in size) more likely to absorb
small quantities of water. This can lead to partial
dissolution at the surface of the metal oxide particles – in
the case of alumina particles, water-soluble aluminium
hydroxide species are formed. Fine particles in contact with
each other can fuse completely or partially to form what
are known as hard or brittle agglomerates. Hard
agglomerates cannot be easily separated without fracturing
the newly formed assembly of individual particles, unlike
brittle agglomerates, which are easier to break up with
sonication or agitation (simple or vigorous).
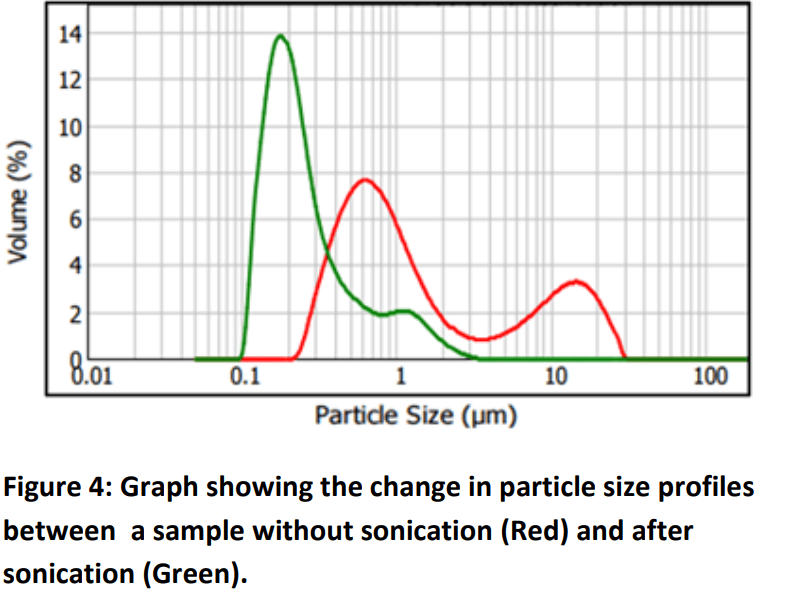
However, it is important to point out that most of the
aggregates that are observed at industrial level are easy to
break. In this regard, figure 4 shows a powder with
aggregates that are quickly broken into the constituent
particles after a quick sonication.
Conclusion
The key to obtaining reproducible and representative
results of particle size distribution by wet laser diffraction is
to prepare a good, stable dispersion of the particle
suspension (colloidal system).
A well-dispersed suspension is a colloid in which the
minimum particle size has been reached and operationally
defined by a constant (minimum) particle size distribution.
This results in complete de-agglomeration of the metal
oxide suspension particles (wet process), which will enable
us to obtain identical or similar results with or without
sonication.
If the addition of a surfactant, mechanical agitation or
heating is not sufficient to separate the fragile
agglomerates, ultrasonic energy can be applied.
A time study using ultrasound can be useful to determine
the sample preparation parameters when fragile
agglomerates are present and when they do not reflect the
main objective of the particle size analysis, we can crosscheck internal methods with other orthogonal techniques
such as:
– Particle size analysis using DLS (Dynamic Light Scattering)
– Centrifugal liquid sedimentation analysis (CLSA)
– Particle Imaging Analysis (PIA) to accurately measure the
particle size distribution of agglomerated particles.
– Nanotrac Nanoparticle analyser.