Are Solid State Batteries Where the Industry Needs Them to Be?
Mark Fearns – Marketing at Advanced Energy Minerals
Introduction
The topic of solid-state batteries is one which excites the interest of both the scientific and public spheres
due to their potential for improved performance in vehicles, enhanced safety against conventional lithium-ion batteries and reduced environmental impact. Against the leading lithium-ion batteries of today, they
boast prospective advantages such as the reduced risk of catastrophic thermal runaway by removal of a
flammable electrolyte and significantly enhanced energy densities of over 400 Wh/ kg.
Though the liquid electrolyte battery industry has seen a meteoric rise with the development and
implementation of lithium-ion batteries, at a rate almost unprecedented in peacetimes, the solid-state
sector is yet to yield the same level of widespread commercial success despite the growing scientific and
public interest. In this issue of the AEM Newsletter, we explore where the solid-state battery status is at
and what still needs to be done before we can all enjoy safe, 1000km range vehicles.
Technological Features
A conventional lithium-ion battery (Figure 1) is composed of a lithium-based inorganic complex as a
cathode active material (CAM), such as lithium iron phosphate (LiFPO4) or lithium oxides containing cobalt
and/or manganese; an anode typically based on graphite or silicon-carbon materials; a polymeric
separator material to regulate the ionic flow and prevent a short circuit; and an electrolyte (liquid or
polymer based) used to facilitate the flow of lithium ions.
An archetypal room-temperature solid-state battery (SSB) configuration principally differs with the
removal of the liquid phase electrolyte, instead relying on a solid or gel-like media to facilitate the flow of
ions. The solid electrolyte (SE) is found in both the cathodic component of the battery as well as the
separator, encompassing a broad variety of chemistries from ceramics to polymers, sulfides and even
halides. Interestingly, one of the most prominent anodes for SSBs is in fact, lithium metal, which is paired
with lithium nickel oxides, carbonates, and nickel manganese cobalt (NMC) complexes – such as those
developed by Prologium and QuantumScape, who target electric vehicles.
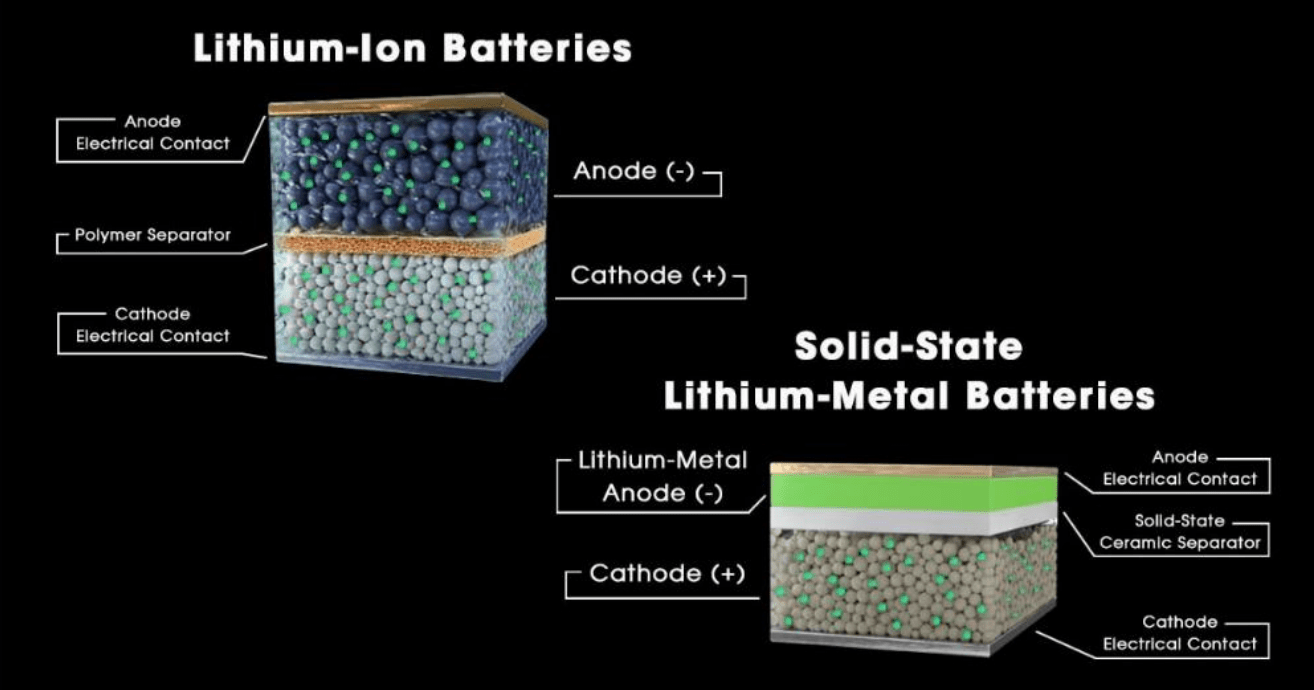
Figure 1: A diagram of a general lithium-ion battery and lithium metal SSB structure. Source: Flash® Battery srl
In SSBs the cathodic component of the battery makes up the majority of the cell volume at around 70%,
with the separator at around 10% (ideally as low as possible) and the anode making up around 20% by
volume. Contrastingly a liquid lithium-ion system contains closer to equal volumetric ratios between the
cathode and anode.
Lithium metal, sodium and silicon-based anodes show excellent promise as high-performance anodes for
fast charging and minor capacity fading, with the potential for a higher lithium-ion transference number
than liquid electrolyte systems, though both experience significant volume changes during their charge
and discharge cycles. A lithium carbon composite may provide the best balance of maintaining high
capacity and specific energy, though published research into this approach is very limited. Higher
volumetric and gravimetric capacities than carbon-based electrodes.
Challenges
The primary challenges facing present-day solid-state battery configurations are the changes in volume of
the battery components during charging and discharging, limitations in achieving the ionic conductivities
found in lithium-ion batteries and interfacial phenomena known as solid-electrolyte interphase (SEI) – an
intermediary phase between electrodes which may add resistivity, worsen diffusion of ions or even
increased flammability to a solid-state battery.
Anodes and cathodes in SSBs often are faced with interfacial resistance which can give rise to unwanted
porosity, requiring sintering or coating to overcome this phenomenon. This is exacerbated by the
expansion and contraction of electrodes about the interface which further leads to raised porosity and
dendrite formation.
Lithium batteries rely on cobalt and nickel which prompts the need for sodium SSBs. Sodium runs into the
issue of having enhanced rate of cathodic degradation compared to lithium batteries, generating the
requirement for coatings on the cathode active materials (CAM) to facilitate adequate ion transport
without degrading the cathode. Further development into SEs which are more compatible with room
temperature sodium systems, such as hydroborates which have excellent electrochemical stability. Sodium
based SSBs have enjoyed notable success in the form of the molten sodium ZEBRA battery, based on
sodium nickel chloride, in addition to sodium sulfur batteries, have achieved commercial status for energy
storage applications, though have yet to achieve utilisation in electric vehicles owing to their size and
operational temperature of above 110 oC. Prominent companies include NGK and Altech Batteries.
Current SSBs struggle to reliably match the ionic conductivity and diffusion kinetics achievable by liquid
electrolytes (LEs). As a reference, the ionic conductivities of lithium and sodium hexafluorophosphates in
mixture of ethylene carbonate and dimethyl carbonate is in the range of 5 – 10 mS/ cm, with 10 mS/ cm
achievable by many modern configurations. Whilst at one research team (Y. Kato et al.) reported an ionic
conductivity of 25 mS/ cm for a lithium metal system at room temperature, using a lithium germanium
phosphorous sulfide (LGPS) lithium super ionic conductor (LISICON). Significant developments into the
room temperature ionic conductivity of SEs have been made in recent times, however, particularly in the
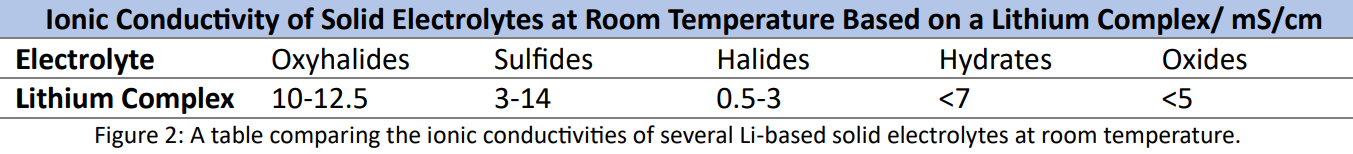
case of lithium SEs. A publication on the use of lithium oxyhalides (Y. Tanaka et al.) (Figure 2) shows
significant promise whilst giving examples of the ionic conductivities against other Li-based SEs.
Another prominent challenge facing the commercialisation of SSBs are the effects of volumetric expansion
during operation. Solid electrolytes based on oxides may incur less expansion than sulfide-based systems,
but as indicated they generally have reduced ionic conductivity against their sulfide counterparts. The risk
of volumetric expansion, and thus destruction of the battery cell, is often mitigated in lab conditions by
applying linear pressure to maintain the structure of the cell; a procedure not feasible in the event of fullscale commercialisation of this technology particularly if the target application is electric vehicles. The
requirements for stack pressure therefore present a serious design consideration for the manufacturers of
sulfide-based SEs.
Similarly with regards to the change from liquid electrolytes to solid, the porosity of the cathode is of
paramount importance. A cathode which has a very high packing density of cathode active materials
(CAMs) will in theory have a higher energy density, though it will suffer tortuosity in the transport of ions
generating a bottleneck and impeding ionic flow. This will require pore optimisation or may demand the
need for an even more conductive solid electrolyte. If the cathode is too porous then the energy density
of the cell will decrease and may cease to be competitive.
Safety
Solid-state batteries are heralded as a safter option to lithium-ion batteries in the public sphere. The
removal of a flammable electrolyte, typically composed of an organic solvent, does offer the possibility of
reduced risk of thermal runaway effects, though a full assessment of the safety of SSBs is not yet fully
developed.
Solid-electrolyte interphase (SEI) may have a reduced level of diffusivity in a solid system due to solid
kinetics. The flammability of such SEI phenomena must still be investigated, though in one case a research
team experienced the ignition of a lithium aluminium germanium phosphate (LAGPO) species at 200 oC
about the interface between the SE and the lithium metal anode, despite being under inert conditions in
a glove box.
Dendrite formation has been previously described as an unwanted phenomenon in battery configurations.
From a health and safety perspective, the dendrites are newly exposed areas of anode which propagate
beyond their intended volume and, in effect, may exponentially raise the rate of reaction of the
electrochemical processed within a battery, generating heat and eventually thermal runaway.
Incompatibilities between the separator and the anode may raise porosity about the solid electrolyte
interphase which can provide a platform for dendrite growth to form if not properly mitigated. Sintering
of ceramic-based SEs is a measure taken to alleviate the risk of dendrite growth about the SEI, however,
this raises production cost in large scale implementation.
Towards the Future
The development of battery technology has been groundbreaking in the past 20 years. Lithium-ion systems
have now achieved full commercialisation and show no signs of slowing down, barring legislative or
industrial mining changes. Solid state batteries present the next pass of the baton in the promethean
development of sustainable, high power energy systems. SSBs have also made significant developmental
progress, and show excellent promise even against LIB systems, though to achieve the same level of
commercialisation, further development is needed based on publicly available information.
Ensuring high ionic conductivity is one of the primary challenges facing SSBs. Alternative materials,
processing, and coating technologies (alumina, ceramics) may help improve ionic conductivity without
raising electrical conductivity, with even greater benefits if they can mitigate unwanted SEI effects.
Lithium anodes are currently the highest performing in terms of energy density under room temperature
operation, but the dendrite formation and inherent flammability of the metal are challenges in their own
right. Further development into hybrid anodes or the use of silica and carbon may help improve in this
regard.
Ensuring a competitive packing density of cathode active materials will be a challenge for the industry as
it strives to compete with itself.
The solid-state battery market is not where it needs to be today, but it may exceed where it needs to be
in the near future.


